Topic: Chemical properties of elements.
Plan:
1. Properties of metals and their compounds ( Chapters I and II subgr.)
1.1 IA elements
1.2 Elements of IIA groups general characteristics.
2. Chemical properties of рelements.
2.1 IIIA elements groups general characteristics.
2.2 Elements of IVA groups general characteristics.
2.3 VA elements groups general characteristics.
2.4 VIA elements groups general characteristics.
2.5 Elements of VIIA groups general characteristics.
2.6 Elements of VIIIA groups general characteristics.
3.Hydrogen
Alkali metals elements of the main subgroup of group I of D. I. Mendeleev’s Periodic Table of Chemical Elements: lithium Li, sodium Na, potassium K, rubidium Rb, cesium Cs and francium Fr. These metals are called alkaline metals because most of their compounds are soluble in water. In Slavic, “leach” means “dissolve,” which determined the name of this group of metals. When alkali metals are dissolved in water, soluble hydroxides called alkalis are formed. G. Davy was the first to obtain free potassium and sodium in 1807.
In the outer electron layer, alkali metal atoms have one electron each. In the second outer electron layer, the lithium atom contains two electrons, and the atoms of the remaining alkali metals have eight electrons each. Having only one electron in the outer electron layer, located at a relatively large distance from the nucleus, the atoms of these elements give up this electron quite easily, i.e., they are characterized by low ionization energy. The singly charged positive ions formed in this case have a stable electronic structure of the corresponding noble gas (lithium ion structure of a helium atom, sodium ion neon atom, etc.). The ease of giving up external electrons characterizes the elements under consideration as the most typical representatives of metals: metallic properties are especially pronounced in alkali metals.
The identical structure of not only the outer, but also the penultimate electronic layer of the atoms of all alkali metals, except lithium, determines the great similarity of the properties of these elements. At the same time, an increase in the nuclear charge and the total number of electrons in an atom when moving from top to bottom through a subgroup creates some differences in their properties. As in other groups, these differences manifest themselves mainly in an increase in the ease of donation of valence electrons and an increase in metallic properties with increasing atomic number.
|
Properties of alkali metals |
||||||||
|
Atomic |
Name, |
Metal |
Ionic |
Potential |
EO |
p, |
tpl, |
t kip, |
|
Lithium Li |
0,152 |
0,078 |
5,32 |
0,98 |
0,53 |
1347 |
||
|
Sodium Na |
0,190 |
0,098 |
5,14 |
0,93 |
0,97 |
|||
|
Potassium K |
0,227 |
0,133 |
4,34 |
0,82 |
0,86 |
|||
|
Rubidium Rb |
0,248 |
0,149 |
4,18 |
0,82 |
1,53 |
|||
|
Cesium Cs |
0,265 |
0,165 |
3,89 |
0,79 |
1,87 |
|||
Chemical properties of alkali metals. Due to the high chemical activity of alkali metals in relation to water, oxygen, and nitrogen, they are stored under a layer of kerosene. To carry out a reaction with an alkali metal, a piece the right size carefully cut off with a scalpel under a layer of kerosene, in an argon atmosphere the surface of the metal is thoroughly cleaned of the products of its interaction with air, and only then the sample is placed in a reaction vessel.
1. Interaction with water. Important property of alkali metalsthem high activity in relation to water. Lithium reacts most calmly (without explosion) with water:
When a similar reaction is carried out, sodium burns with a yellow flame and a small explosion occurs. Potassium is even more active: in this case the explosion is much stronger, and the flame is colored purple. 2. Interaction with oxygen. The products of combustion of alkali metals in air have different composition depending on the activity of the metal.
Only lithium burns in air to form an oxide of stoichiometric composition:
When sodium burns, Na2O2 peroxide is mainly formed with a small admixture of NaO2 superoxide:
The combustion products of potassium, rubidium and cesium contain mainly superoxides: ![]()
To obtain sodium and potassium oxides, mixtures of hydroxide, peroxide or superoxide with an excess of metal are heated in the absence of oxygen: 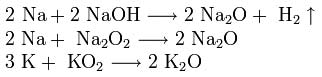
The following pattern is characteristic of oxygen compounds of alkali metals: as the radius of the alkali metal cation increases, the stability of oxygen compounds containing peroxide ion O22 and superoxide ion O2- increases.
Heavy alkali metals are characterized by the formation of fairly stable ozonides with the composition EO3. All oxygen compounds have different colors, the intensity of which deepens in the series from Li to Cs:
|
Formula |
Color |
|
Li2O |
White |
|
Na2O |
White |
|
K2O |
Yellowish |
|
Rb2O |
Yellow |
|
Cs2O |
Orange |
|
Na2O2 |
Light |
|
KO 2 |
Orange |
|
RbO2 |
Dark- |
|
CsO2 |
Yellow |
Alkali metal oxides have all the properties of basic oxides: they react with water, acidic oxides and acids: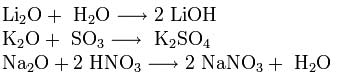
Peroxides and superoxides exhibit the properties of strong oxidizing agents:
Peroxides and superoxides interact intensively with water, forming hydroxides:
3. Interaction with other substances. Alkali metals react with many nonmetals. When heated, they combine with hydrogen to form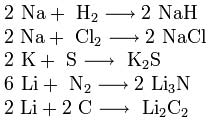 hydrides, with halogens, sulfur, nitrogen, phosphorus, carbon and silicon to form, respectively, halides, sulfides, nitrides, phosphides, carbides and silicides:
hydrides, with halogens, sulfur, nitrogen, phosphorus, carbon and silicon to form, respectively, halides, sulfides, nitrides, phosphides, carbides and silicides:
When heated, alkali metals are capable of reacting with other metals, forming intermetallic compounds. Alkali metals react actively (explosively) with acids.
Alkali metals dissolve in liquid ammonia and its derivatives - amines and amides:
When dissolved in liquid ammonia, an alkali metal loses an electron, which is solvated by ammonia molecules and gives the solution a blue color. The resulting amides are easily decomposed by water to form alkali and ammonia:
Alkali metals interact with organic substances, alcohols (to form alcoholates) and carboxylic acids (to form salts):
4. Qualitative definition alkali metals. Since the ionization potentials of alkali metals are small, when the metal or its compounds are heated in a flame, the atom is ionized, coloring the flame a certain color:
|
Flame coloring with alkali metals |
|
|
Carmine red |
|
|
Yellow |
|
|
Violet |
|
|
Whitish pink |
|
|
Purple-red |
|
Preparation of alkali metals
1. To obtain alkali metals, they mainly use electrolysis of melts of their halides, most often chlorides that form natural minerals:
cathode: Li+ + e → Lianode: 2Cl- 2e → Cl22. Sometimes to obtain alkali metals they carry out
electrolysis of melts of their hydroxides:
cathode: Na+ + e → Naanode: 4OH- 4e → 2H2O + O2 Since alkali metals in the electrochemical voltage series are located to the left of hydrogen, their electrolytic production from salt solutions is impossible; in this case, the corresponding alkalis and hydrogen are formed.
In nature alkali metals found exclusively in the form of compounds. Sodium and potassium are constant components many very common silicates. Of the individual sodium minerals, the most important is table salt (NaCl). sea water and in certain areas of the earth's surface it forms huge deposits of so-called rock salt under a layer of alluvial rocks (in the USSR - Solikamsk, Artemoven, Iletsk, etc.). IN upper layers Similar deposits sometimes contain accumulations of potassium salts [in the form of minerals sylvinite (KClNaCl), carnallite (KCl MgCl2 6H2 O), etc.], which serve as the main source of compounds of this element. Only a few natural accumulations of potassium salts of industrial importance are known. The most important of them is the Solikamsk field in the USSR.
A number of minerals are known for lithium [for example, spodumene LiAl (SiO3) 2 ], but their accumulations are rare. Rubidium and cesium occur almost exclusively as impurities in other alkali metals. Traces of francium are always found in uranium ores.
Sodium and potassium compounds are of great importance for life. Suffice it to recall that a person annually consumes 510 kg of NaCl. In the same way, plants need potassium salts. In this regard, about 90% of all mined potassium compounds are used to fertilize soils. The remaining 10%, as well as huge quantities of various sodium compounds, are used in industry. So far, lithium derivatives and very limited Rb and Cs compounds have found only relatively small applications.
In a free state alkali metals can be isolated by electrolysis of their molten chloride salts. Sodium is of primary practical importance, the annual world production of which is more than 50 thousand tons.
The main subgroup of group II includes elements b epu liy, magnesium, calcium, strontium, barium and radium. All these elements, except beryllium, have pronounced metallic properties. Available and they represent silver Rhysto-white substances, harder than alkali metals, with rather high melting points. In terms of density, all of them, except radium, belong to light metals.
The first two members of the subgroup under consideration occupy a somewhat special position in it, differing in many respects from the other four elements. B erillium according to some of its own quality is approaching aluminum.
All isotopes of the last element of the subgroup, radium, are radioactive. Long-lived isotope 226 Ra formerly used in radiotherapy; it has now been replaced by cheaper isotopes of other elements produced in nuclear reactors.
In the outer electron layer, atoms of elements of this subgroup have two electrons; in the second outer layer, beryllium has two electrons, and the remaining elements have eight.
Simple substances formed by atoms of these elements are metals. Lithium, sodium, potassium, rubidium, cesium and francium are called alkali metals because their hydroxides are alkalis. Calcium, strontium and barium are called alkaline earth metals. The chemical activity of these substances increases as the atomic radius increases. Of the chemical properties of these metals, the most important are their reducing properties. Alkali metals are the strongest reducing agents. Metals of group IIA elements are also quite strong reducing agents. All of them (except beryllium) react with water (magnesium when boiled):
2M + 2H 2 O = 2M aq + 2OH aq + H 2,M + 2H 2 O = M 2 + 2OH + H 2.
In the case of magnesium, calcium and strontium, due to the low solubility of the resulting hydroxides, the reaction is accompanied by the formation of a precipitate:
M 2 + 2OH = Mg(OH) 2
Alkali metals react with most nonmetals: 2M + H 2 = 2MH (when heated), 4M + O 2 = 2M 2 O (M Li), 2M + Cl 2 = 2MCl (under normal conditions), 2M + S = M 2 S (when heated).
Of the alkali metals, when burned in oxygen, the usual oxide forms only lithium. The remaining alkali metals form peroxides (M 2 O 2 ) or superoxides (MO 2
compounds containing a superoxide ion with a formal charge 1 e). Like alkali metals, metals of group IIA elements react with many non-metals, but under more severe conditions: M + H 2 = MH 2 (when heated; except beryllium),
2M+O 2
= 2MO (under normal conditions; Be and Mg when heated), M + Cl 2 = MCl 2 (under normal conditions), M + S = MS (when heated). Unlike alkali metals, they form ordinary oxides with oxygen. Only magnesium and beryllium react calmly with acids; the rest simple substances very violently, often with explosion. Beryllium reacts with concentrated solutions of alkalis: Be + 2OH+ 2H 2 O = 2 + H 2
In accordance with their position in the voltage series, only beryllium and magnesium react with salt solutions; the remaining metals in this case react with water. Being strong reducing agents, alkali and alkaline earth metals reduce many less active metals from their compounds, for example, when heated, the following reactions occur: 4Na + MnO 2 = 2Na 2 O + Mn; 2Ca + SnO 2 = 2CaO + Sn. The industrial method of production common to all alkali metals and group IIA metals is electrolysis of molten salts.
In addition to beryllium, the oxides of all the elements under consideration are basic oxides, and hydroxides are strong bases (in beryllium these compounds are amphoteric, magnesium hydroxide is a weak base). The strengthening of the basic properties of hydroxides with an increase in the atomic number of an element in a group is easily observed in the series of hydroxides of group IIA elements. Be(OH) 2
amphoteric hydroxide, Mg(OH) 2
weak base, Ca(OH) 2, Sr(OH) 2 and Ba(OH) 2 strong bases, but with increasing atomic number their solubility increases, and Ba(OH) 2
can already be classified as alkalis.
All alkaline earth metals are found (in varying quantities) in nature. Due to their high chemical activity, all of them are not found in a free state. The most common alkaline earth metal is calcium, the amount of which is 3.38% (by weight of the earth’s crust). It is slightly inferior to magnesium, the amount of which is 2.35% (of the mass of the earth’s crust). Barium and strontium are also common in nature, accounting for 0.05 and 0.034% of the mass of the earth’s crust, respectively. Beryllium is a rare element, the amount of which is 6 × 10−4% of the mass of the earth's crust. As for radium, which is radioactive, it is the rarest of all alkaline earth metals, but it is always found in small quantities in uranium ores. In particular, it can be isolated from there chemically. Its content is 1×10−10% (of the mass of the earth’s crust)/
The p-elements of group III of D.I. Mendeleev’s periodic table include: boron B, aluminum Al, gallium Ga, indium In and thallium Tl. Electronic configuration atoms ns2 np1 .
Below are compared some constants that characterize the properties of atoms of p-elements of the group under consideration and the corresponding metallic substances:
The properties of group III p-elements are affected by d-compression (Al is located in periodic table in the small III period, and Ga, In and Tl in large periods immediately after the d-elements). Thus, from Al to Ga the atomic radius decreases slightly, and the first ionization potential increases. In addition, f-compression also affects the properties of thallium atoms. That is why the radius of the Tl atom is close to the radius of the In atom, and the ionization energy is slightly higher.
Bor. In accordance with the electronic structure of the atom (1s2 2s2 2p1), boron can be monovalent (one unpaired electron at the 2p energy sublevel). However, boron is most characteristic of compounds in which it is trivalent (when an atom is excited, there are three unpaired electrons in the 2s and 2p energy sublevels).
The free 2p orbital in the excited boron atom determines the acceptor properties of many of its compounds, in which three covalent bonds are formed according to the exchange covalent mechanism (for example, Br3). These compounds are prone to the addition of particles with electron-donating properties, i.e., to the formation of another covalent bond according to the donor-acceptor mechanism. For example:
BBr3 + Br- = [BBr4 ]-
Two isotopes of boron are known: 105 B (19.6%) and 11 5 B (80.4%). The nuclei of atoms of the isotope (105 V) easily absorb neutrons:
105 V + 1 0 n = 4 2 He + 7 5 Li
The ability of boron to absorb neutrons determines its use in nuclear energy: control rods of nuclear reactors are made from boron-containing materials.
Boron crystals are black; they are refractory (mp 2300 °C), diamagnetic, and have semiconductor properties (band gap ΔE = 1.55 eV). The electrical conductivity of boron, like other metals, is low and increases slightly with increasing temperature.
At room temperature boron is chemically inert and interacts directly only with fluorine; When heated, boron is oxidized by chlorine, oxygen and some other non-metals. For example:
4B + 3O2 = 2B2 O3
2В + ЗСl2 = 2ВСl3
In compounds with non-metals, the oxidation state of boron is +3; all these compounds are covalent.
Boron trioxide B2 O3 is a crystalline substance (mp 450 °C, boiling point 2250 °C), characterized by high values of enthalpy and Gibbs energy of formation. When interacting with water, B2 O3 transforms into boric acid:
B2 O3 + ZH2 O = 2H3 BO3
H3 BO3 is a very weak (Kd ≈ 10-9) monobasic acid. The electrolytic dissociation of H3 BO3 with the elimination of only one H+ ion is explained by the previously described acceptor properties of boron: the free 2p orbital of the boron atom is provided to the electron donor OH-, formed during the dissociation of H2 O molecules. The process proceeds according to the scheme
H3 BO3 + H2 O = H[B(OH)4 ] = H+ + [B(OH)4]-
The complex anion [B(OH)4]- has a tetrahedral structure (sp3 - hybridization of electronic orbitals). The acceptor properties of boron in compounds with oxidation state + 3 are also manifested in the chemistry of its halides. For example, the reactions
BF3 + F- = -
BF3 + NH3 =
in which the chemical bond between BF and F- or NH3 is formed by a donor-acceptor mechanism. The property of boron halides to be electron acceptors determines their wide application as catalysts in reactions of synthesis of organic compounds.
Boron does not interact directly with hydrogen, but with metals it forms borides, usually non-stoichiometric compounds Me4B, Me2B, MeB, Me3B4, MeB2 and MeB6.
Boron hydrides (boranes) are very poisonous and have very bad smell. They are obtained indirectly, most often
when reacting chemically active borides with acids or boron halides with alkali metal hydrides:
6MgB2 + 12HCl = H2 + 8B + B4 H10 + 6MgCl2
8BF3 + 6LiH = B2 H6 + 6LiBF4
The simplest compound of boron with hydrogen BH3 does not exist under ordinary conditions, sp2 - Hybridization of electron orbitals in the boron atom leads to coordination unsaturation of the BH3 particle, as a result of which two such particles combine into a diborane molecule: 2BH3 = B2 H6 (ΔG0 298 = -127 kJ/ mole).
In diborane B2H6, boron is in a state of 5p3 hybridization, and for each boron atom one of the four hybrid orbitals is empty, and the other three are overlapped by s-orbitals of hydrogen atoms. Bonds between BH3 groups in the B2H6 molecule are formed as a hydrogen bond due to a shift in electron density from one hydrogen atom of the BH3 group to an empty orbital of another BH3 group. Other boranes are also known, which can be represented by two rows Bn Hn+4 and Bn Hn+6.
S-metal borides are reactive and are often used to produce borane mixtures by treatment with acids. Most borides of d- and f-metals are heat-resistant, very hard, and chemically stable. They are widely used directly in the form of alloys for the manufacture of jet engine parts and gas turbine blades. Some borides are used to make cathodes of electronic devices.
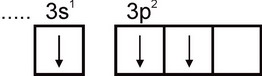
Aluminum. The electronic configuration of an aluminum atom is expressed by the formula 1s2 2s2 2p6 3s2 3p1. The outermost electron layer of an atom has one unpaired electron:
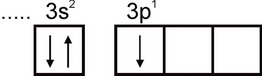
Therefore, aluminum can exhibit a valency equal to one. However, this valence is not typical for aluminum. In all stable compounds, the oxidation state of aluminum is +3. A valency of three corresponds to the excited state of the Al atom:
In terms of its abundance, aluminum ranks fourth among all elements (after O, H and Si) and is the most common metal in nature. The bulk of aluminum is concentrated in aluminosilicates: feldspars, micas, etc.
Aluminum silver-white, lightweight and extremely ductile metal with high thermal and electrical conductivity.
Aluminum is chemically active; It reacts with chlorine and bromine at room temperature, and with iodine when heated or in the presence of water as a catalyst. At 800 °C aluminum reacts with nitrogen, and at 2000 °C with carbon. Aluminum exhibits a high chemical affinity for oxygen (ΔG0 298 = -1582 kJ/mol):
2Al + 0.502 = Al2 O3, ΔH0 298 = -1650 kJ/mol
In air, aluminum is covered with a very durable, thin (10-8 m) oxide film, which somewhat weakens the metallic luster of aluminum. Thanks to the oxide film, the aluminum surface acquires high corrosion resistance. This is primarily manifested in the indifference of aluminum to water and water vapor. Due to the formation of a protective film, aluminum is resistant to concentrated nitric and sulfuric acids. These acids passivate aluminum in the cold. The tendency to passivation makes it possible to increase the corrosion resistance of aluminum by treating its surface with strong oxidizing agents (for example, K2 Cr2 O7) or using anodic oxidation. In this case, the thickness of the oxide film increases to 3·10-5 m. When high temperatures the strength of the protective film decreases sharply. If mechanical impact remove the oxide film, aluminum becomes extremely reactive. It reacts vigorously with water and aqueous solutions of acids and alkalis, displacing hydrogen and forming cations or anions. The interaction of aluminum with acid solutions proceeds according to the reaction equation
Al + 6H2 O + 3H+ = [Al(H2 O)6 ]3+ + 0.5H2
and with alkali solutions
Al + 3H2 O + OH- = [Al(OH)4 ]- + 0.5H2
Aluminum cations and anions easily transform into each other when the pH of the solution changes:
[Al(H2 O)6 ]3+ + 4OH- = [Al(OH)4 ]- + 6H2 O
[Al(OH)4 ]- + 4H+ + 2H2 O = [Al(H2 O)6 ]3+
Mixed compounds can also form in solution, for example [Al(H2 O)5 (OH)]2+ , [Al(H2 O)4 (OH)2 ]+ , [Al(H2 O)3 (OH)3 ]. The latter easily (especially when heated) dehydrates and turns into hydroxide Al(OH)3:
[Al(H2 O)3 (OH)3 ] = Al(OH)3 + 3H2 O
The widest use of aluminum in technology is based on its valuable physical and chemical properties and widespread in the earth's crust. Due to its high electrical conductivity (4·10-5 Ohm-1 cm-1) and low density, it is used for the manufacture of electrical wires. The high ductility of aluminum makes it possible to make the thinnest foil from it, which is used in capacitors and replaces lead in cable sheaths with aluminum. Due to their non-magnetizability, aluminum alloys are used in radio engineering.
The bulk of aluminum is used to produce light alloys: duralumin (94% Al, the rest Cu, Mg, Mn, Fe and Si), silumin (85 x 90% Al, 10 x 14% Si, the rest Na), etc. Aluminum is used in addition, as an alloying additive to alloys to impart heat resistance to them. Aluminum and its alloys occupy one of the main places as structural materials in aircraft construction, rocketry, mechanical engineering, etc. The corrosion resistance of aluminum (especially anodized) significantly exceeds the corrosion resistance of steel. Therefore, its alloys are used as structural materials and in shipbuilding. With d-elements, aluminum forms chemical compounds - intermetallides (aluminides): NiAl, Ni3 Al, CoAl, etc., which are used as heat-resistant materials. Aluminum is used in aluminothermy to produce a number of metals and for welding using the thermite method. Aluminothermy is based on the high affinity of aluminum for oxygen. For example, in a reaction proceeding according to the equation
8Al + 3Fe3 O4 = 4Al2 O3 + 9Fe
About 3500 kJ of heat is released and temperatures reach 3000 °C.
Aluminum oxide is known in the form of several modifications. The most stable is α-Al2 O3. This modification is found in the earth's crust in the form of the mineral corundum, from which grinding discs and emery powders are prepared. The use of corundum as an abrasive material is based on its high hardness, second only to the hardness of diamond, SiC carborundum and BN borazone. By fusing Al2 O3 with Cr2 O3 one gets artificial rubies. They are used to make support stones in precision mechanisms. IN lately artificial rubies are used in quantum generators (lasers). Products made from Al2O3 are used as refractories and dielectrics.
Aluminum hydroxide Al(OH)3 polymer compound. It has a layered crystal lattice. Each layer consists of Al(OH)6 octahedra (Fig. 1); There is a hydrogen bond between the layers. Aluminum hydroxide obtained by exchange reaction is a gelatinous white precipitate, highly soluble in acids and alkalis. When standing, the sediment “ages” and loses its chemical activity. When calcined, the hydroxide loses water and turns into Al2O3 oxide. One of the forms of dehydrated hydroxide, aluminum gel, is used in technology as an adsorbent.
Aluminum compounds zeolites, related to aluminosilicates, are of extremely great interest. Their composition can be expressed by the general formula Meh Eu O2y ·nH2 O, where MeCa or Na (less commonly Ba, Sr, K); E Si and Al in a variable ratio. Zeolite crystals have channels into which H2O molecules can penetrate. The water content in zeolites varies widely depending on the water vapor pressure. Zeolites are capable of exchanging the water they contain for other liquids (alcohol, etc.). With gentle heating, water is gradually removed from the zeolites. But even complete dehydration does not lead to the destruction of zeolite crystals. Ca2+ or Na+ cations in zeolites form a diffusion layer; they are not fixed in the crystal lattice, but, together with water, are located in the voids of the crystal. This explains the presence of cation exchange properties in zeolites that are important for technical purposes. The ability to replace some cations with others allows the use of zeolites as ion exchangers.
A number of artificial zeolites are used as so-called molecular sieves. Crystals of the latter are characterized by the presence of narrow channels with a diameter from 3·10-10 to 13·10-10 m. Molecular sieves absorb substances whose molecules can enter these holes.
For example, a molecular sieve with a hole diameter of 3.5 x 10-10 m can absorb H2, O2, N2 molecules, but does not absorb larger CH4 or Ar molecules. Using molecular sieves, you can separate hydrocarbons, dry gases, etc.
Gallium, indium and thallium in the form of simple substances are low-melting silvery-white metals. The physical and chemical properties of Ga, In and Tl differ markedly from the properties of Al, despite the similarity of the electronic structure of the external energy level of the atoms of the elements under consideration.
This is apparently due to the difference in the electronic structure of the pre-external energy level of Al atoms: (n-1)s2 (n-1)p6 on the one hand and Ga, In and Tl atoms on the other: (n-1)s2 (n- 1)р6 (n-1)d10 .
The oxidation state of gallium and indium in stable compounds is +3. The oxidation state of + 1 is more typical for thallium. Thallium compounds, in which the oxidation state of the metal is +3, are strong oxidizing agents.
Gallium has a wide temperature range of existence of the liquid state. The low melting point (about 30 °C) and high boiling point (2205 °C) make it possible to use liquid gallium for the manufacture of pressure gauges.
Indium uniformly reflects light waves of all wavelengths and is therefore used in precision instrumentation for the manufacture of mirrors. In addition, In is part of some low-melting alloys.
Thallium is also introduced into some alloys, mainly alloys with tin and lead (acid-resistant, bearing).
The oxides Ga2 O3, In2 O3 and Tl2 O3 are practically insoluble in water. A noticeable increase in the basic properties in the series Ga2 O3 In2 O3 Tl2 O3 is manifested in the increasing solubility of oxides in acids.
The hydroxides Ga(OH)3, In(OH)3, Tl(OH)3 are as insoluble in water as the oxides. The white precipitate of Ga(OH)3 is soluble equally in acids and alkalis, and the red-brown Tl(OH)3 is soluble only in acids.
Gallium and indium form binary compounds of type AIII BV with p-elements of group V of the periodic system (for example, GaP, GaAs, InSb, etc.). In the vast majority of type AIII BV compounds, the electronic orbitals are sp3-hybridized; the crystal lattices of these compounds have a structure characterized by a tetrahedral arrangement of chemical bonds. Many of these diamond-like compounds are semiconductors. They are used as a material for AC rectifiers, sensors, thermoelectric generators, etc.
Carbon, silicon, germanium, tin and lead form the main subgroup of group IV. The outer energy levels of group IV p-elements contain four electrons (ns2np2 configuration), of which two are paired s-electrons and two unpaired p-electrons.
In an unexcited state, elements of this subgroup exhibit a valency of two. Upon transition to an excited state, accompanied by the transition of one of the s-electrons of the outer level to a free cell of the p-sublevel of the same level, all electrons of the outer layer become unpaired, and the valence increases to 4.
Sulfur, selenium, tellurium and polonium also have external level 6 electrons (s 2 p 4 ), but they all have an unfilled d-level, so they can have up to 6 unpaired electrons and exhibit oxidation states of -2, +4 and +6 in compounds.
The pattern of changes in the activity of these elements is the same as in the subgroup of halogens: tellurides are most easily oxidized, then selenides and sulfides. Of the oxygen compounds of sulfur, the most stable are sulfur (VI) compounds, and for tellurium - tellurium (IV) compounds. Selenium compounds occupy an intermediate position.
In elements of the chromium subgroup, the d-level is filled, therefore, at the s-level of their atoms there are 1 (for chromium and molybdenum) or 2 (for tungsten) electrons. All of them exhibit a maximum oxidation state of +6, but molybdenum, and especially chromium, are characterized by compounds in which they have a lower oxidation state (+4 for molybdenum and +3 or +2 for chromium). Chromium(III) compounds are very stable and similar to aluminum compounds.
All metals of the chromium subgroup are widely used.
Molybdenum was first obtained by K. V. Scheele in 1778. It is used in the production of high-strength and toughness steels used for the manufacture of weapon barrels, armor, shafts, etc.
Due to its ability to evaporate at high temperatures, it is of little use for making filaments, but it has good ability to fuse with glass, so it is used to make tungsten filament holders in incandescent lamps.
Tungsten was also discovered by K.V. Scheele in 1781. It is used to produce special steels. The addition of tungsten to steel increases its hardness, elasticity and strength. Together with chromium, tungsten gives steel the ability to maintain hardness at very high temperatures, which is why such steels are used to make cutters for high-speed lathes.
Pure tungsten has the highest melting point among metals (3370 degrees C), therefore it is used for the manufacture of filaments in incandescent lamps. Tungsten carbide has very high hardness and heat resistance and is the main integral part refractory alloys.
The nitrogen subgroup consists of five elements: nitrogen, phosphorus, arsenic, antimony and bismuth. These are p-elements of the V group of the periodic system of D. I. Mendeleev.At the outer energy level, the atoms of these elements contain five electrons, which have the ns2np3 configuration and are distributed as follows:
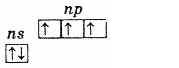
Therefore, the highest oxidation state of these elements is +5, the lowest -3, and +3 is also characteristic. The presence of three unpaired electrons at the outer level indicates that in an unexcited state the atoms of the elements have a valence of 3. The outer level of the nitrogen atom consists of only two sublevels 2s and 2p. The atoms of the remaining elements of this subgroup have external energy levels there are vacant d-sublevel cells. Consequently, one of the s-electrons of the outer level can, upon excitation, move to the d-sublevel of the same level, which leads to the formation of 5 unpaired electrons.
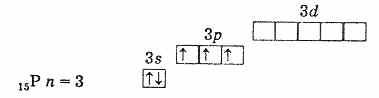
outer electron shell of phosphorus (unexcited atom)
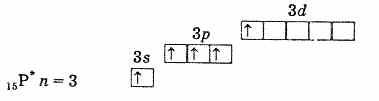
outer electron shell of an excited phosphorus atom. Thus, phosphorus, arsenic, antimony and bismuth in an excited state have 5 unpaired electrons, and their valence in this state is 5. Excite an electron in a nitrogen atom in a similar way is impossible due to the absence of a d-sublevel at the second level. Therefore, nitrogen cannot be pentavalent, but it can form a fourth covalent bond by the donor-acceptor mechanism due to the lone electron pair 2s2. Another process is also possible for the nitrogen atom. When one of the two 2s electrons is removed, nitrogen becomes a singly charged tetravalent ion N+.

From nitrogen to bismuth, the atomic radii increase, and the ionization potentials decrease. The reducing properties of neutral atoms increase from N to Bi, and the oxidizing properties weaken (see Table 21).
With hydrogen, nitrogen, phosphorus and arsenic form polar compounds RH3, exhibiting a negative oxidation state of -3. RH3 molecules have a pyramidal shape. In these compounds, the bonds of elements with hydrogen are stronger than in the corresponding compounds of elements of the oxygen subgroup and especially the halogen subgroup. Therefore, hydrogen compounds of elements of the nitrogen subgroup in aqueous solutions do not form hydrogen ions.
With oxygen, elements of the nitrogen subgroup form oxides of the general formula R2O3 and R2O5. Oxides correspond to acids HRO2 and HRO3 (and ortho acids H3RO4, except nitrogen). Within the subgroup, the nature of the oxides changes as follows: N2O3 acid oxide; Р4О6 weakly acidic oxide; As2O3 amphoteric oxide with predominant acidic properties; Sb2O3 amphoteric oxide with predominant basic properties; Bi2O3 basic oxide. Thus, the acidic properties of oxides of composition R2O3 and R2O5 decrease with increasing atomic number of the element. As can be seen from the table. 21, within the subgroup from nitrogen to bismuth, non-metallic properties decrease and metallic properties increase. In antimony, these properties are expressed equally; in bismuth, metallic properties predominate; in nitrogen, nonmetallic properties predominate. Phosphorus, arsenic and antimony form several allotropic compounds.
The sixth group of the periodic table of elements consists of two subgroups: the main group - oxygen, sulfur, selenium, tellurium and polonium - and the secondary group - chromium, molybdenum and tungsten. In the main subgroup, a selenium subgroup is distinguished (selenium, tellurium and polonium), a secondary subgroup is called the chromium subgroup. All elements of the main subgroup, except oxygen, can add two electrons, forming electronegative ions.
Elements of the main subgroup have six electrons (s2p4) in the outer electronic level. Oxygen atoms have two unpaired electrons and no d-level. Therefore, oxygen exhibits mainly an oxidation state of (2 and only in compounds with fluorine +2. Sulfur, selenium, tellurium and polonium also have six electrons in their outer level (s2p4), but they all have an unfilled d-level, so they can have up to six unpaired electrons and exhibit oxidation states of 2, +4 and +6 in compounds.
The pattern of changes in the activity of these elements is the same as in the subgroup of halogens: tellurides are most easily oxidized, then selenides and sulfides. Of the oxygen compounds of sulfur, the most stable are sulfur (VI) compounds, and for tellurium, tellurium (IV) compounds. Selenium compounds occupy an intermediate position.
Selenium and tellurium, as well as their compounds with certain metals (indium, thallium, etc.) have semiconductor properties and are widely used in radio electronics. Selenium and tellurium compounds are very toxic. They are used in glass industry for producing colored (red and brown) glasses.
In elements of the chromium subgroup, the d-level is filled, therefore, at the s-level of their atoms there are one (for chromium and molybdenum) or two (for tungsten) electrons. All of them exhibit a maximum oxidation state of +6, but molybdenum, and especially chromium, are characterized by compounds in which they have a lower oxidation state (+4 for molybdenum and +3 or +2 for chromium). Chromium(III) compounds are very stable and similar to aluminum compounds. All metals of the chromium subgroup are widely used.
Molybdenum was first obtained by K.V. Scheele in 1778. It is used in the production of high-strength and toughness steels used for the manufacture of weapon barrels, armor, shafts, etc. Due to the ability to evaporate at high temperatures, it is of little use for the manufacture of filaments , but has a good ability to fuse with glass, so it is used to make tungsten filament holders in incandescent lamps.
Tungsten was also discovered by K.V. Scheele in 178! d. It is used to produce special steels. The addition of tungsten to steel increases its hardness, elasticity and strength. Together with chromium, tungsten gives steel the ability to maintain hardness at very high temperatures, which is why such steels are used to make cutters for high-speed lathes. Pure tungsten has the highest melting point among metals (3370 (C), therefore it is used for the manufacture of filaments in incandescent lamps. Tungsten carbide is very hard and heat-resistant and is the main component of refractory alloys.
The elements included in group VII of the periodic table are divided into 2 subgroups: the main one - the halogen subgroup - and the secondary one - the manganese subgroup. Hydrogen is also placed in the same group, although its atom has a single electron at the outer valence level and should be placed in group I.
However, hydrogen has very little in common with both the elements of the main subgroup - the alkali metals, and the elements of the secondary subgroup - copper, silver and gold. At the same time, like halogens, it adds an electron in reactions with active metals and forms hydrides that have some similarities with halides.
The subgroup of halogens includes fluorine, chlorine, bromine, iodine and astatine. The first 4 elements are found in nature, the last one is obtained artificially and therefore has been studied much less than the other halogens. The word "halogen" means salt-forming. The elements of the subgroup received this name due to the ease with which they react with many metals, forming salts.
All halogens have an outer electron shell structure s 2p5 . Therefore, they easily accept an electron, forming a stable noble gas electron shell (s 2 r 6 ). Fluorine has the smallest atomic radius in the subgroup; for the rest it increases in the series F< Cl < Br < I < Аt и составляет соответственно 133; 181; 196; 220 и 270 нм. В таком же порядке уменьшается сродство атомов элементов к электрону.
Halogens - very active elements. They can take electrons not only from atoms that easily give them up, but also from ions and even displace other halogens, less active, from their compounds. For example, fluorine displaces chlorine from chlorides, bromine from bromides, and iodine from iodides.
Of all the halogens, only fluorine, which is in period II, does not have an unfilled d-level. For this reason, it cannot have more than 1 unpaired electron and exhibits only a valence of -1. In the atoms of other halogens, the d-level is not filled, which allows them to have a different number of unpaired electrons and exhibit the -1, +1, +3, +5 and +7 valences observed in the oxygen compounds of chlorine, bromine and iodine.
The manganese subgroup includes manganese, technetium and rhenium. Unlike halogens, elements of the manganese subgroup have only 2 electrons on the outer electronic level and therefore do not exhibit the ability to attach electrons, forming negatively charged ions.
Manganese is abundant in nature and widely used in industry.
Technetium is radioactive, it is not found in nature, but was obtained artificially (first by E. Segre and C. Perrier, 1937). This element is formed due to the radioactive decay of uranium. Rhenium is one of the trace elements. It does not form independent minerals, but is found as a companion of some minerals, especially molybdenum.
It was discovered by V. and I. Noddak in 1925. Alloys with small additions of rhenium have increased resistance to corrosion. The addition of rhenium to alloys increases their mechanical strength.
This property of rhenium allows it to be used instead noble metal iridium. Platinum-platinum-rhenium thermocouples perform better than platinum-platinum-iridium thermocouples, but they cannot be used at very high temperatures because a volatile Re compound is formed 2 O 7 .
The main subgroup of the eighth group of the periodic table consists of noble gaseshelium, neon, argon, krypton, xenon and radon. These elements are characterized by very low chemical activity, which gives rise to calling them noble, or inert, gases. They only form compounds with other elements or substances with difficulty; chemical compounds of helium, neon and argon have not been obtained. Atoms of noble gases are not combined into molecules, in other words, their molecules are monatomic.
The noble gases end each period of the system of elements. Except for helium, they all have eight electrons in the outer electron layer of the atom, forming a very stable system. The electron shell of helium, consisting of two electrons, is also stable. Therefore, noble gas atoms are characterized by high ionization energies and, as a rule, negative electron affinity energies.
For a long time it was believed that noble gas atoms were generally incapable of forming chemical bonds with atoms of other elements. Only relatively unstable molecular compounds of noble gases were known, for example, hydrates
Ag-6N 2 0, Kg-6N 2 0, Xe-6N 2 0, formed by the action of compressed noble gases on crystallizing supercooled water. These hydrates belong to the clathrate type; valence bonds do not arise during the formation of such compounds. The formation of clathrates with water is favored by the presence of crystal structure ice of numerous cavities.
However, during last decades it was found that krypton, xenon and radon are capable of combining with other elements and, above all, with fluorine. So, ; fluorides are obtained by direct interaction of noble gases with fluorine (by heating or in an electric discharge) KrF 2, XeF 2, KrF 4, XeF 4 and RnF 4 . All of them are crystals that are stable under ordinary conditions. Xenon derivatives were also obtained in the oxidation state; +6 hexafluoride XeF 6 , XeO3 trioxide, Xe(OH)b hydroxide. The last two compounds exhibit acidic properties; so, reacting with alkalis, they form saltsxenonic acid, for example: Xe0 3 + Ba(OH) 2 BaXe0 4 + H 2 0.
Derivatives; xenon(VI)strong oxidizing agents. However, when exposed to even stronger oxidizing agents, it is possible to obtain compounds in which xenon has an oxidation state of -4-8. Of these compounds, octafluoride is known XeF 8, Xe0 4 tetroxide and oxohexafluoride XeOF 6.
The higher chemical activity of krypton, xenon and radon compared to the first members of the group of noble gases is explained by the relatively low ionization potentials of their atoms. For krypton, xenon and radon, these values are close to the ionization potentials of some other elements (for example, the ionization potential of the nitrogen atom is 14.53 IN, chlorine atom 12.97 V).
Greatest practical application Argon, neon and helium are found.
Hydrogen (lat. Hydrogenium),H, chemical element, first by atomic number in the periodic table of Mendeleev; atomic mass 1.0079. Under normal conditions, Hydrogen is a gas; has no color, smell or taste.
Historical information.The works of chemists of the 16th and 17th centuries repeatedly mentioned the release of flammable gas when acids act on metals. In 1766, G. Cavendish collected and studied the gas released, calling it “combustible air.” Being a supporter of the phlogiston theory, Cavendish believed that this gas was pure phlogiston. In 1783, A. Lavoisier, through analysis and synthesis of water, proved the complexity of its composition, and in 1787 he identified “combustible air” as a new chemical element (Hydrogen) and gave it modern name hydrogene (from the Greek hydor - water and gennao - I give birth), which means “giving birth to water”; this root is used in the names of Hydrogen compounds and processes with its participation (for example, hydrides, hydrogenation). The modern Russian name "Hydrogen" was proposed by M. F. Solovyov in 1824.
Distribution of Hydrogen in nature.Hydrogen is widespread in nature; its content in the earth's crust (lithosphere and hydrosphere) is 1% by mass and 16% by number of atoms. Hydrogen is part of the most common substance on Earth - water (11.19% of Hydrogen by mass), in the composition of compounds that make up coal, oil, natural gases, clays, as well as animal and plant organisms (that is, in the composition of proteins, nucleic acids , fats, carbohydrates and others). In the free state, Hydrogen is extremely rare, in small quantities it is found in volcanic and other natural gases. Minor amounts of free Hydrogen (0.0001% by number of atoms) are present in the atmosphere. In near-Earth space, Hydrogen in the form of a flow of protons forms the internal (“proton”) radiation belt of the Earth. In space, Hydrogen is the most abundant element. In the form of plasma, it makes up about half the mass of the Sun and most stars, the bulk of the gases of the interstellar medium and gaseous nebulae. Hydrogen is present in the atmosphere of a number of planets and in comets in the form of free H 2, methane CH 4, ammonia NH 3, water H 2 O, radicals such as CH, NH, OH, SiH, PH, etc. In the form of a flow of protons, Hydrogen is part of the corpuscular radiation of the Sun and cosmic rays.
Isotopes, atom and molecule of Hydrogen.Ordinary Hydrogen consists of a mixture of 2 stable isotopes: light Hydrogen, or protium ( 1 H), and heavy Hydrogen, or deuterium ( 2 H, or D). In natural hydrogen compounds per 1 atom 2 H accounts for an average of 6800 atoms 1 H. A radioactive isotope with mass number 3 is called superheavy Hydrogen, or tritium ( 3 H, or T), with soft β-radiation and half-life T½ = 12.262 years. In nature, tritium is formed, for example, from atmospheric nitrogen under the influence of cosmic ray neutrons; in the atmosphere it is negligible (4 10-15 % of the total number of Hydrogen atoms). Extremely unstable isotope obtained 4 H. Mass numbers of isotopes 1 N, 2 N, 3 N and 4 H, 1, 2, 3 and 4, respectively, indicate that the nucleus of a protium atom contains only one proton, deuterium - one proton and one neutron, tritium - one proton and 2 neutrons, 4 H - one proton and 3 neutrons. The large difference in the masses of Hydrogen isotopes causes a more noticeable difference in their physical and chemical properties than in the case of isotopes of other elements.
The Hydrogen atom has the simplest structure among the atoms of all other elements: it consists of a nucleus and one electron. The binding energy of an electron with a nucleus (ionization potential) is 13.595 eV. The neutral hydrogen atom can also add a second electron, forming a negative ion H- in this case, the binding energy of the second electron with a neutral atom (electron affinity) is 0.78 eV. Quantum mechanics allows us to calculate all possible energy levels of the Hydrogen atom, and therefore give a complete interpretation of its atomic spectrum. The Hydrogen atom is used as a model atom in quantum mechanical calculations of the energy levels of other, more complex atoms.
Molecule Hydrogen H 2 consists of two atoms joined by a covalent bond chemical bond. The energy of dissociation (that is, decay into atoms) is 4.776 eV. The interatomic distance at the equilibrium position of the nuclei is 0.7414 Å. At high temperatures, molecular Hydrogen dissociates into atoms (the degree of dissociation at 2000°C is 0.0013, at 5000°C 0.95). Atomic Hydrogen is also formed in various chemical reactions(for example, the effect of Zn on hydrochloric acid). However, the existence of Hydrogen in the atomic state lasts only short time, atoms recombine into molecules H 2 .
Physical properties of Hydrogen.Hydrogen is the lightest of all known substances (14.4 times lighter than air), density 0.0899 g/l at 0°C and 1 atm. Hydrogen boils (liquefies) and melts (solidifies), respectively, at -252.8°C and -259.1°C (only helium has more low temperatures melting and boiling). The critical temperature of Hydrogen is very low (-240°C), so its liquefaction is fraught with great difficulties; critical pressure 12.8 kgf/cm 2 (12.8 atm), critical density 0.0312 g/cm 3 . Of all gases, Hydrogen has the highest thermal conductivity, equal to 0.174 W/(m K) at 0°C and 1 atm, that is, 4.16 10-4 cal/(s·cm·°С). Specific heat capacity of Hydrogen at 0°C and 1 atm C p 14.208 kJ/(kg K), that is, 3.394 cal/(g °C). Hydrogen is slightly soluble in water (0.0182 ml/g at 20°C and 1 atm), but well soluble in many metals (Ni, Pt, Pa and others), especially in palladium (850 volumes per 1 volume of Pd). The solubility of Hydrogen in metals is related to its ability to diffuse through them; Diffusion through a carbon alloy (for example, steel) is sometimes accompanied by destruction of the alloy due to the interaction of Hydrogen with carbon (so-called decarbonization). Liquid Hydrogen is very light (density at -253°C 0.0708 g/cm 3 ) and fluid (viscosity at -253°C 13.8 spoise).
Chemical properties of Hydrogen.In most compounds, Hydrogen exhibits a valence (more precisely, oxidation state) +1, like sodium and other alkali metals; it is usually considered as an analogue of these metals, heading group I of the periodic system. However, in metal hydrides, the Hydrogen ion is negatively charged (oxidation state -1), that is, Na hydride+H- built like Na chloride+ Cl - . This and some other facts (proximity physical properties Hydrogen and halogens, the ability of halogens to replace Hydrogen in organic compounds) give grounds to classify Hydrogen also in group VII of the periodic table. Under ordinary conditions, molecular Hydrogen is relatively little active, directly combining only with the most active of non-metals (with fluorine, and in the light with chlorine). However, when heated, it reacts with many elements. Atomic Hydrogen has increased chemical activity compared to molecular Hydrogen. With oxygen, hydrogen forms water:
H 2 + 1/2 O 2 = H 2 O
with the release of 285.937 kJ/mol, that is, 68.3174 kcal/mol of heat (at 25°C and 1 atm). At normal temperatures the reaction proceeds extremely slowly, above 550°C - with explosion. The explosive limits of the hydrogen-oxygen mixture are (by volume) from 4 to 94% H 2 , and the hydrogen-air mixture - from 4 to 74% H 2 (mixture of 2 volumes H 2 and 1 volume O 2 called detonating gas). Hydrogen is used to reduce many metals, as it removes oxygen from their oxides:
CuO + H 2 = Cu + H 2 O,
Fe 3 O 4 + 4H 2 = 3Fe + 4H 2 O, etc.
With halogens Hydrogen forms hydrogen halides, for example:
H 2 + Cl 2 = 2HCl.
At the same time, Hydrogen explodes with fluorine (even in the dark and at - 252°C), reacts with chlorine and bromine only when illuminated or heated, and with iodine only when heated. Hydrogen reacts with nitrogen to form ammonia:
ZN 2 + N 2 = 2NH 3
only on a catalyst and at elevated temperatures and pressures. When heated, Hydrogen reacts vigorously with sulfur:
H 2 + S = H 2 S (hydrogen sulfide),
much more difficult with selenium and tellurium. Hydrogen can react with pure carbon without a catalyst only at high temperatures:
2H 2 + C (amorphous) = CH 4 (methane).
Hydrogen reacts directly with some metals (alkali, alkaline earth and others), forming hydrides:
H 2 + 2Li = 2LiH.
Of great practical importance are the reactions of Hydrogen with carbon monoxide (II), in which various organic compounds are formed, depending on temperature, pressure and catalyst, for example HCHO, CH 3 HE and others. Unsaturated hydrocarbons react with Hydrogen, becoming saturated, for example:
C n H 2n + H 2 = C n H 2n+2.
The role of Hydrogen and its compounds in chemistry is exceptionally great. Hydrogen determines the acidic properties of so-called protic acids. Hydrogen tends to form a so-called hydrogen bond with some elements, which has a decisive influence on the properties of many organic and inorganic compounds.
Obtaining Hydrogen.The main types of raw materials for the industrial production of Hydrogen are natural flammable gases, coke oven gas and oil refining gases. Hydrogen is also obtained from water by electrolysis (in places with cheap electricity). The most important methods for producing Hydrogen from natural gas are the catalytic interaction of hydrocarbons, mainly methane, with water vapor (conversion):
CH 4 + H 2 O = CO + ZN 2,
and incomplete oxidation of hydrocarbons with oxygen:
CH 4 + 1/2 O 2 = CO + 2H 2
The resulting carbon monoxide (II) also undergoes conversion:
CO + H 2 O = CO 2 + H 2.
Hydrogen produced from natural gas is the cheapest.
Hydrogen is isolated from coke oven gas and oil refining gases by removing the remaining components of the gas mixture, which liquefy more easily than Hydrogen during deep cooling. Electrolysis of water is carried out with direct current, passing it through a solution of KOH or NaOH (acids are not used to avoid corrosion of steel equipment). In laboratories, Hydrogen is obtained by electrolysis of water, as well as by the reaction between zinc and hydrochloric acid. However, more often they use ready-made hydrogen in cylinders.
Application of Hydrogen.Hydrogen began to be produced on an industrial scale at the end of the 18th century for filling balloons. Currently, Hydrogen is widely used in the chemical industry, mainly for the production of ammonia. A major consumer of Hydrogen is also the production of methyl and other alcohols, synthetic gasoline and other products obtained by synthesis from Hydrogen and carbon monoxide (II). Hydrogen is used for the hydrogenation of solid and heavy liquid fuels, fats and others, for the synthesis of HCl, for the hydrotreatment of petroleum products, in welding and cutting of metals with an oxygen-hydrogen flame (temperature up to 2800°C) and in atomic-hydrogen welding (up to 4000°C) . Hydrogen isotopes - deuterium and tritium - have found very important applications in nuclear energy.
Chemical properties of elementsPage 13
Chemistry is a science that studies chemical elements, the simple and complex substances they form (composition, structure, properties), their transformations and the laws to which these transformations obey. It is divided into inorganic, organic, physical, analytical, colloidal, etc. Modern chemistry is connected with other sciences, as a result of which border areas of science arise: biochemistry, agrochemistry, cosmochemistry, radiochemistry, etc. The achievements of modern chemistry are a stimulus intensive growth chemical industry, play important role in scientific and technological progress of all industries national economy. Chemistry plays an important role in solving the most pressing and promising problems modern society(increasing the efficiency and safety of artificial fertilizers to increase the yield of agricultural products and the problem of synthesis of food products from non-food raw materials; development of ocean sources of raw materials; development and creation of new energy sources; synthesis of new substances and compositions necessary to solve problems in the future; protection environment). See No. 2, p334.
The object of study in chemistry is chemical elements and their compounds. A chemical element is a collection of atoms with equal charge cores. In turn, an atom is the smallest particle of a chemical element that retains all its chemical properties. Thus, each chemical element corresponds to a certain type of atom. See No. 3, p.11.
A molecule is the smallest particle of an individual substance capable of independent existence, possessing its basic chemical properties and consisting of identical or different atoms. Molecules can be one-, two-, or polyatomic. They are constituent particles of matter. If the molecules consist of identical atoms, then the substance is called simple or elementary, for example He, Ar, H2, O2, O3, S4, P4. A simple substance is a form of existence of a chemical element in a free state. See No. 3, p11 p12.
If the molecule of a substance consists of different atoms, then the substance is called a complex (or chemical compound), for example CO, H2O, NH3, H3PO4. Any substance is characterized by a certain composition (the nature and number of atoms in its molecule), structure (spatial arrangement of atoms in the molecule) and certain physical and chemical properties. See No. 3, p.12.
The chemical properties of a substance characterize its ability to participate in chemical reactions, that is, in the processes of converting some substances into others. To understand these properties, it is necessary to know the composition and structure of substances. See No. 3, p.12.
The entire periodic table can be divided into metals, nonmetals and amphoteric substances. Metals- simple substances characterized by the ability to donate electrons located at the external energy level (valence electrons) and transform into positively charged ions. Almost all metals have high electrical and thermal conductivity, the ability to reflect light waves well (which determines their shine and opacity), and plasticity. In the solid state they usually have a crystalline structure. The connection between atoms in a metal is carried out by valence electrons, which move freely in the crystal lattice formed by positively charged metal ions. Of the 107 elements in the periodic table, 83 elements are metals. Many of the performance properties of metals depend not only on their chemical properties, but also on the structure that they acquire as a result of the methods of production and subsequent processing. This creates opportunities for a wide change in the properties of metals and makes them the most important structural, electrical, mechanical and other materials. Today, metals are widely used in various fields of technology. Nonmetals- simple substances that are not malleable, metallic shine, are poor conductors of heat and electricity. Non-metal atoms are predominantly characterized by the ability to attach electrons, i.e. turn into negatively charged ions. Nonmetals include 22 elements: H, B, C, Si, N, P, As, O, S, Se, Te, halogens and noble gases. Oxides of non-metals are acidic in nature; they correspond to oxygen-containing acids. Amphoteric substances- substances that tend to exhibit both acidic and basic properties. An amphoteric substance, reacting, for example, with a strong base, can exhibit acidic properties, while at the same time, the same substance, reacting with a strong acid, can exhibit basic properties. See No. 2, p273, p279, p225.
Let's consider the periodic table of chemical elements. It was created on the basis of the periodic law. The table consists of 7 periods and 8 groups.
Periods are horizontal rows of the table, they are divided into large and small. In soap periods there are 2 elements (1st period), or 8 elements (2nd and 3rd periods), in large periods there are 18 elements (4th and 5th) or 32 elements (6th period) . The 7th period is not over yet. Any period begins with a typical metal and ends with a typical non-metal and noble gas. See No. 1, p271.
Vertical columns are called groups of elements. Each group is divided into two subgroups: main and secondary. A subgroup is a collection of elements that are chemical analogues. Often elements of a subgroup have highest degree oxidation corresponding to the group number. See No. 1, p271.
In the main subgroups, the chemical properties of elements can vary widely
"Chemistry. 9th grade." O.S. Gabrielyan (GDZ)
Characteristics of chemical elements. Periodic law of Mendeleev
Question 1.
Mendeleev's periodic law is one of the fundamental laws of chemistry. It can be argued that all modern chemistry built on it. He explains the dependence of the properties of atoms on their structure, generalizes this dependence for all elements, dividing them into various groups, and also predicts their properties depending on the structure and structure depending on the properties.
There are other laws that have explanatory, generalizing and predictive functions. For example, the law of conservation of energy, the law of light refraction, Mendel's genetic law, the law universal gravity etc.
Question 2.
Guided by the periodic table, we will find an element with the desired arrangement of electrons at energy levels (2 and 5). This element is nitrogen: N: 1s 2 2s 2 2p 3.
This element forms a simple substance - N 2, nitrogen. Hydrogen compound of nitrogen - NH 3, ammonia. The highest nitric oxide is N2O5. This oxide belongs to the acidic oxides, because when dissolved in water it becomes strong nitric acid HNO3.
N 2 O 5 + H 2 O = 2HNO 3;
N 2 O 5 + BaO = Ba(OH) 2;
N 2 O 5 + Ba(OH) 2 = Ba(NO 3) 2 + H 2 O;
Nitrogen in the oxidation state +5 (N +5) has strong oxidizing properties:
2N 2 O 5 + 5C = 2N 2 + 5CO 2.
Question 3.
Previously, the element beryllium was mistakenly attributed to III group. The reason for this was the incorrect determination of the atomic mass of beryllium (instead of 9 it was considered equal to 13.5). DI. Mendeleev suggested that beryllium was in group II, based on the chemical properties of the element. The properties of beryllium were very similar to those of magnesium (Mg) and calcium (Ca), and completely different from those of aluminum (Al). Knowing that the atomic masses of Li and B, neighboring elements to Be, are equal to 7 and 11, respectively, D.I. Mendeleev assumed that the atomic mass of beryllium is 9.
Question 4.
Ca is an atom whose electrons are distributed according to a series
numbers 2, 8, 8, 2.
Element No. 7-N,
element No. 8 - O.
Reaction equations:
3Ca + N 2 = Ca 3 N 2,
2Ca + O 2 = 2CaO.
The reaction products have an ionic bond type.
Nitrogen and oxygen have a molecular structure crystal lattices, and calcium has a metal crystal lattice.
The interaction products - Ca 3 N 2 and CaO - have an ionic crystal lattice structure.
Question 5.
N, P, As, Sb, Bi – strengthening of metallic properties.
The metallic properties of the groups are enhanced.
Question 6.
In the series of elements: N, Mg, Al, Si, P, S, Cl - strengthening of non-metallic properties.
The non-metallic properties of elements increase in periods.
Question 7.
Cl 2 O 7, P 2 O 5, SiO 2, Al 2 O 3, MgO, Na 2 O – decrease in acid properties.
Acid properties increase during periods.
HClO 4, H3PO 4, H 2 SO 4, Al(OH) 3, NaOH – decrease in acid properties.
Question 8.
B 2 O 3 , BeO, Li 2 O – increase in basic properties.
B(OH) 3, Be(OH) 2, LiOH - increase in basic properties.
B(OH) 3 – weak acid;
Be(OH) 2 - weak base;
LiOH is a strong base.
Question 9.
The periodic table of elements reflects the relationship of chemical elements. The atomic number of an element is equal to the charge of the nucleus, and numerically it is equal to the number of protons. The number of neutrons contained in the nuclei of one element, in contrast to the number of protons, can be different. Atoms of one element whose nuclei contain different number neutrons are called isotopes.
Each chemical element has several isotopes (natural or artificially obtained). The atomic mass of a chemical element is equal to the average value of the masses of all its natural isotopes, taking into account their abundance.
With the discovery of isotopes, the charges of nuclei, rather than their atomic masses, began to be used to distribute elements across the periodic table.
Question 10.
This happens because the properties of elements and their compounds do not depend on the total number of electrons, but only on the number of valence electrons that are located in the last layer. The number of valence electrons changes periodically, therefore, the properties of elements also change periodically.
Question 11.
1. The properties of chemical elements and the substances formed by them are periodically dependent on the relative atomic masses of the elements.
2. The properties of chemical elements and the substances formed by them periodically depend on the charge of the atomic nuclei of the elements.














